Lenacapavir is a cutting-edge HIV drug that targets the virus’s protective shell, stopping it from multiplying. Its long-acting injection lasting up to 6 months offers convenience and hope, especially for those resistant to other treatments. Fewer doses have a big impact
Lenacapavir is an antiviral medication used for the treatment of HIV-1 infection. It belongs to a class of drugs known as capsid inhibitors. It is designed to disrupt the viral capsid, an essential protein structure that protects HIV’s genetic material. By targeting this structure, lenacapavir can prevent HIV replication and reduce the viral load in the body.
Key Features of Lenacapavir
- Mechanism of Action:
Lenacapavir inhibits the capsid protein of HIV, which is crucial for multiple stages of the virus’s lifecycle, including assembly, disassembly, and replication. - Dosage Form:
It is available as an injectable (subcutaneous injection) and an oral tablet. The long-acting injectable form is particularly noteworthy for reducing the frequency of medication administration. - Indications:
Approved for use in heavily treatment-experienced adults with multidrug-resistant HIV-1, especially when other treatments have failed or are not well-tolerated. - Administration Schedule:
The long-acting injectable formulation allows for dosing every 6 months, offering significant convenience for patients. - Side Effects:
Common side effects may include injection site reactions, nausea, fatigue, or headache. Serious side effects, though rare, may involve hypersensitivity reactions. - Combination Therapy:
Lenacapavir is often used as part of combination antiretroviral therapy (ART) since HIV treatment typically requires multiple drugs to prevent resistance.
Detailed Overview of the HIV Capsid
The HIV capsid is a cone-shaped protein shell that encloses the viral RNA genome and essential enzymes required for replication, such as reverse transcriptase, integrase, and protease. It plays a critical role in protecting the viral components and mediating several key stages of the virus’s lifecycle.
1. Structure of the HIV Capsid
The capsid is primarily composed of capsid protein (CA), also known as p24. CA is a product of the Gag polyprotein precursor. The capsid is formed through the self-assembly of CA monomers into a lattice of hexamers and pentamers, creating the distinct conical shape.
The majority of the capsid is made of CA hexamers, providing the structural foundation. These hexamers form the curved lattice required for the conical structure. Located at the tips of the cone, pentamers help close the structure, maintaining its integrity.
Each CA monomer consists of two domains. The N-terminal domain (NTD) is essential for hexamer and pentamer formation, as well as for interacting with host proteins. On the other hand, the C-terminal domain (CTD) is important for stabilizing the capsid structure and facilitating protein-protein interactions.
2. Functions of the HIV Capsid
The capsid is not just a protective shell; it plays an active role in the virus’s lifecycle. The HIV capsid plays a vital role in ensuring the virus’s survival and replication within the host cell. It provides protection by shielding the viral RNA and enzymes from detection by the host’s immune system during their transport to the nucleus. Once inside the host cell, the capsid undergoes a process known as uncoating, where it gradually disassembles to release the viral RNA and enzymes needed for replication.
The capsid also facilitates nuclear import by interacting with host proteins, which help transport the viral pre-integration complex (PIC) into the nucleus. After reaching the nucleus, the capsid supports integration by aiding in the incorporation of viral DNA into the host genome, a critical step for establishing infection. These functions underscore the capsid’s importance in the HIV lifecycle.
3. Host Protein Interactions
The capsid interacts with several host cell proteins at different stages of the lifecycle. These interactions are crucial for the virus to evade immune defenses, transport its genome, and replicate efficiently.
The HIV capsid relies on several host proteins to facilitate its journey through the cell and support its replication. One key protein is Cyclophilin A (CypA), which stabilizes the capsid during its transport through the cytoplasm, ensuring it remains intact until it reaches the nucleus. Once near the nucleus, the capsid interacts with nucleoporins (NUPs), proteins that are part of the nuclear pore complex. These interactions help guide the capsid and its genetic material into the nucleus.
Additionally, Transportin-SR2 (TNPO3) plays a crucial role in aiding the nuclear entry of the pre-integration complex, which includes the capsid, viral enzymes, and genetic material. Once inside the nucleus, the capsid relies on LEDGF/p75, a host protein that anchors the viral integration machinery to the host chromatin, ensuring the integration of HIV DNA into the host genome is both precise and efficient. These interactions highlight the capsid’s dynamic relationship with host proteins, which is essential for the HIV lifecycle.
4. Role in Immune Evasion
The capsid’s design also allows HIV to avoid detection by the host’s innate immune system. For example, TRIM5α binds to the viral capsid once the retrovirus enters the cytoplasm of the target cell. It then compartmentalizes the capsid core and disrupts its disassembly process. However, the capsid’s design of HIV can evade antiviral restriction factors like TRIM5α in humans. Additionally, the interaction with Cyclophilin A helps HIV resist innate immune sensing.
5. Drug Target Potential
Given its critical role in the HIV lifecycle, the capsid has emerged as a promising target for antiretroviral drugs like lenacapavir. These drugs work by interfering with the capsid’s essential functions, which can effectively block the nuclear import of the viral genome, preventing it from reaching the host cell’s nucleus. Additionally, they can disrupt the proper assembly of the capsid during the formation of new viral particles, rendering these particles non-infectious. Furthermore, drugs like lenacapavir can destabilize the capsid, causing premature uncoating and halting the virus’s replication process at an early stage. These mechanisms highlight the potential of capsid-targeting therapies in controlling HIV infection.
6. Evolutionary Significance
The HIV capsid is finely tuned for optimal interaction with human host proteins. This evolutionary adaptation ensures efficient replication and immune evasion, making it a key focus for both virology research and therapeutic development.
The HIV capsid is not just a passive container; it is a dynamic and multifunctional structure that plays a critical role in the virus’s ability to replicate, evade the immune system, and cause disease. Drugs targeting the capsid, like lenacapavir, represent a novel and promising approach to disrupting the HIV lifecycle.
In short, HIV capsid interacts with several host proteins during its lifecycle to facilitate the transport of viral RNA into the nucleus. A key protein in this process is nuclear pore complex (NPC)-associated proteins, particularly Nucleoporin 62 (NUP62) and other nucleoporins. These proteins form the gateway through which the HIV capsid moves to enter the nucleus.
Specific Interaction and Binding Sites on the Capsid
- Cyclophilin A (CypA):
- The HIV capsid binds to Cyclophilin A (CypA) via its cyclophilin-binding loop, a highly conserved region on the capsid. This interaction stabilizes the capsid and is critical for successful transport through the cytoplasm and into the nucleus.
- Nucleoporins (NUPs):
- The capsid also interacts with nucleoporins such as NUP153 and NUP358. These interactions occur at specific regions called phenylalanine-glycine (FG) repeats on the nucleoporins, which facilitate capsid docking and nuclear entry.
- LEDGF/p75:
- During integration, the capsid interacts with lens epithelium-derived growth factor (LEDGF/p75), which helps tether the pre-integration complex to the host chromatin.
- Specific Capsid Binding Sites:
- The capsid protein (CA) has distinct regions important for these interactions, including:
- Hexameric Interface: The structural interface where capsid monomers assemble into hexamers, critical for binding host factors like CypA.
- CypA-Binding Loop: A key loop on the capsid recognized by Cyclophilin A.
- N-terminal and C-terminal domains: Important for structural integrity and binding to various host proteins.
- The capsid protein (CA) has distinct regions important for these interactions, including:
Target Sites of Lenacapavir on the Capsid
Lenacapavir binds to a specific site on the HIV capsid protein, targeting the p24 protein (a core component of the capsid). It binds at the interface between the N-terminal and C-terminal domains of the capsid protein, stabilizing or destabilizing the capsid as needed to block essential interactions with host proteins like NUP62 and Cyclophilin A.
By disrupting these critical interactions, lenacapavir prevents the capsid from completing its function, effectively halting HIV replication at multiple stages.
Dosage Form Of Lenacapavir
Lenacapavir is available in two main dosage forms: an oral tablet and a long-acting injectable, providing flexibility to meet different patient needs. The oral tablet is primarily used as a loading dose at the start of treatment to achieve a therapeutic dose. This initial dosing phase is often combined with other antiretroviral medications to establish a strong therapeutic foundation. Once this phase is complete, patients typically transition to the long-acting injectable form for sustained treatment.
The injectable form is designed for sustained release, with a single dose lasting up to 6 months, making it ideal for long-term HIV management. This eliminates the need for daily medication and offers significant convenience, especially for patients who struggle with adherence to daily oral regimens. By providing consistent drug levels in the body, the injectable form ensures better viral suppression and reduces the risk of treatment failure due to missed doses.
The combination of these two dosage forms allows healthcare providers to tailor treatment plans to individual patient needs, starting with the oral form for rapid initiation and transitioning to the injectable form for long-term control. The long-acting injectable is particularly beneficial for individuals with adherence challenges or those in regions with limited access to healthcare. Overall, lenacapavir’s innovative dosing options represent a significant advancement in HIV therapy, combining flexibility, convenience, and effectiveness.
Dosage and Administration
| Recommended Treatment Regimen for LENACAPAVIR Initiation and Maintenance | |
| Treatment Time | |
| Initiation Option 1 | |
| Day 1 | 927mg by subcutaneous injection (2 X 1.5ml injections) 600 mg orally (2 X 300mg tablet) |
| Day 2 | 600 mg orally (2 X 300mg tablet) |
| Initiation Option 2 | |
| Day 1 | 600 mg orally (2 X 300mg tablet) |
| Day 2 | 600 mg orally (2 X 300 mg tablet) |
| Day 8 | 300 mg orally ( 1 X 300 mg tablet) |
| Day 15 | 927mg by subcutaneous injection (2 X 1.5ml injections) |
| Dosage of LENACAPAVIR Maintenance | |
| Every 6 months (26 weeks) from the date of last injection +/- 2 weeks | 927 mg by subcutaneous injection (2X1.5ml injections) |
Missed dose: If more than 28 weeks since the last injection and clinically appropriate to continue LENACAPAVIR, restart initiation from Day 1, using either Option 1 or Option 2.
LENACAPAVIR (SULENCA) is injected into the abdomen by a healthcare provider.
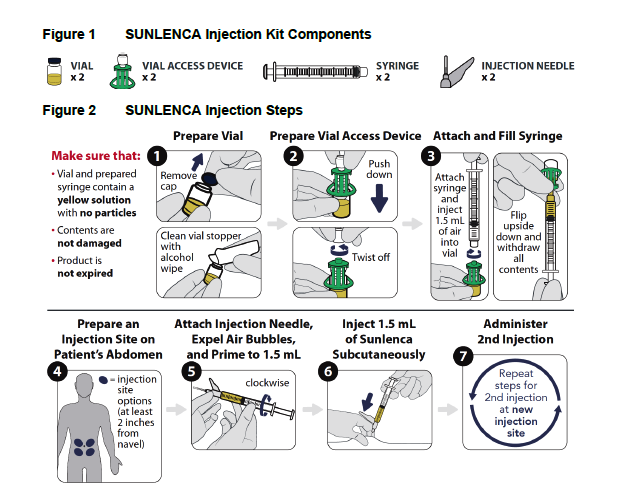
Contraindication of Lenacapavir
Concomitant administration of lenacapavir with strong inducers of CYP3A is contraindicated as the plasma concentration of lenacapavir is decreased, which may result in the loss of therapeutic efficacy and development of resistance to Lenacapavir.
Adverse Effects
Immune Reconstitution Syndrome (IRS)
Immune reconstitution syndrome (IRS) can occur in patients starting combination antiretroviral therapy (cART). As the immune system begins to recover during the early phase of treatment, some individuals may develop an inflammatory reaction to existing opportunistic infections, such as Mycobacterium avium, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis. This reaction may require additional medical evaluation and management.
Autoimmune conditions, such as Graves’ disease, polymyositis, Guillain-Barré syndrome, and autoimmune hepatitis, have also been associated with immune reconstitution. Unlike IRS, the onset of autoimmune disorders can vary widely and may appear months after starting therapy.
Injection Site Reactions with LENACAPAVIR
The administration of lenacapavir can lead to injection site reactions (ISRs), which may require evaluation, appropriate treatment, and follow-up if they become clinically significant. ISRs may present with symptoms such as pain, swelling, redness, nodules, induration, itching, masses, or extravasation. Among these, nodules and indurations at the injection site often take longer to resolve compared to other ISRs.
Clinical studies observed that, after a median follow-up of 553 days, 30% of injection site nodules and 13% of indurations (reported in 10% and 1% of patients, respectively) from the first injections had not completely resolved. While specific measurements and detailed descriptions of ISRs were not consistently reported, most nodules and indurations were found to be palpable but not visible, with a size range of approximately 1 to 4 cm.
The underlying cause of persistent nodules and indurations is not fully understood, but evidence suggests it could be linked to the subcutaneous drug depot formed at the injection site. In certain cases where a skin biopsy of the affected site was conducted, results indicated signs of foreign body inflammation or a granulomatous response.
Nausea and ISRs are the most common adverse reactions.
Stay tuned for our next publication, where we will delve deeper into the clinical implications, patient experiences, and long-term outcomes associated with LENACAPAVIR. We will also talk about its approval in India. Together, we can explore how this innovative treatment continues to shape the future of HIV care.

Hame cahiye